How Do You Know Where to Put the Covalent Bonds
Covalent Bonds
- Page ID
- 1984
Covalent bonding occurs when pairs of electrons are shared by atoms. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full electron shell. Past sharing their outer most (valence) electrons, atoms can make full their outer electron shell and gain stability. Nonmetals volition readily form covalent bonds with other nonmetals in order to obtain stability, and tin can class anywhere between one to 3 covalent bonds with other nonmetals depending on how many valence electrons they posses. Although it is said that atoms share electrons when they grade covalent bonds, they practice non usually share the electrons equally.
Introduction
Simply when two atoms of the same chemical element class a covalent bond are the shared electrons actually shared equally between the atoms. When atoms of dissimilar elements share electrons through covalent bonding, the electron will be drawn more than toward the atom with the college electronegativity resulting in a polar covalent bond. When compared to ionic compounds, covalent compounds normally take a lower melting and boiling betoken, and have less of a tendency to dissolve in water. Covalent compounds can exist in a gas, liquid, or solid state and practise not conduct electricity or heat well. The types of covalent bonds can be distinguished by looking at the Lewis dot structure of the molecule. For each molecule, there are unlike names for pairs of electrons, depending if information technology is shared or not. A pair of electrons that is shared between two atoms is called a bond pair . A pair of electrons that is not shared between two atoms is called a alone pair .
Octet Rule
The Octet Rule requires all atoms in a molecule to have 8 valence electrons--either by sharing, losing or gaining electrons--to go stable. For Covalent bonds, atoms tend to share their electrons with each other to satisfy the Octet Dominion. It requires viii electrons because that is the corporeality of electrons needed to make full a s- and p- orbital (electron configuration); also known as a noble gas configuration. Each atom wants to get as stable equally the noble gases that take their outer valence shell filled because noble gases take a charge of 0. Although it is important to remember the "magic number", 8, note that there are many Octet rule exceptions.
Example: As you can see from the picture below, Phosphorus has but 5 electrons in its outer shell (bolded in red). Argon has a total of 8 electrons (bolded in red), which satisfies the Octet Dominion. Phosphorus needs to proceeds 3 electrons to fulfill the Octet Rule. Information technology wants to exist similar Argon who has a total outer valence beat.
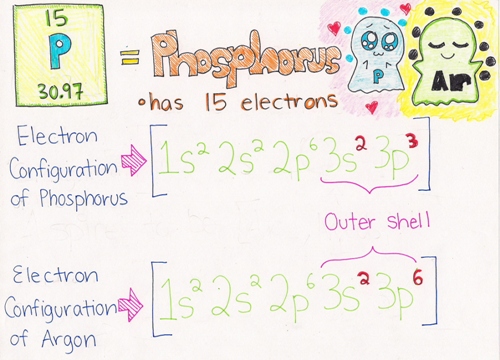
More examples can exist found hither.
Single Bonds
A unmarried bond is when two electrons--one pair of electrons--are shared between two atoms. It is depicted by a single line between the 2 atoms. Although this form of bail is weaker and has a smaller density than a double bond and a triple bond, it is the most stable because information technology has a lower level of reactivity meaning less vulnerability in losing electrons to atoms that want to steal electrons.
Example 1: HCl
Beneath is a Lewis dot structure of Hydrogen Chloride demonstrating a single bond. As nosotros tin can come across from the motion picture below, Hydrogen Chloride has i Hydrogen atom and 1 Chlorine atom. Hydrogen has only 1 valence electron whereas Chlorine has 7 valence electrons. To satisfy the Octet Rule, each cantlet gives out 1 electron to share with each other; thus making a single bail.

Double Bonds
A Double bail is when two atoms share 2 pairs of electrons with each other. Information technology is depicted by two horizontal lines between two atoms in a molecule. This blazon of bond is much stronger than a single bond, but less stable; this is due to its greater amount of reactivity compared to a single bail.
2
Below is a Lewis dot structure of Carbon dioxide demonstrating a double bond. As you can come across from the picture below, Carbon dioxide has a total of i Carbon atom and two Oxygen atoms. Each Oxygen atom has half dozen valence electrons whereas the Carbon atom only has iv valence electrons. To satisfy the Octet Dominion, Carbon needs iv more valence electrons. Since each Oxygen atom has 3 solitary pairs of electrons, they can each share one pair of electrons with Carbon; as a outcome, filling Carbon's outer valence shell (Satisfying the Octet Rule).
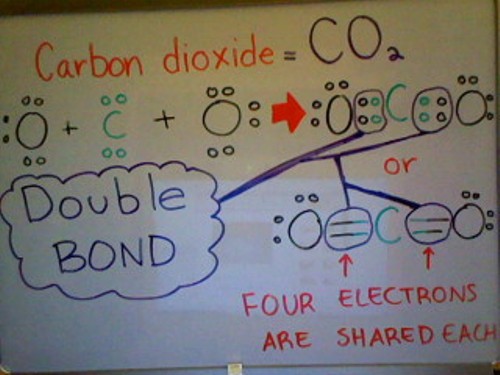
Triple Bond
A Triple bail is when three pairs of electrons are shared betwixt 2 atoms in a molecule. Information technology is the least stable out of the three general types of covalent bonds. It is very vulnerable to electron thieves!
Instance 3: Acetylene
Below is a Lewis dot structure of Acetylene demonstrating a triple bail. As you can see from the moving-picture show beneath, Acetylene has a full of two Carbon atoms and 2 Hydrogen atoms. Each Hydrogen atom has ane valence electron whereas each Carbon cantlet has iv valence electrons. Each Carbon needs 4 more electrons and each Hydrogen needs 1 more electron. Hydrogen shares its simply electron with Carbon to go a total valence shell. At present Carbon has 5 electrons. Because each Carbon cantlet has five electrons--1 single bail and 3 unpaired electrons--the 2 Carbons tin share their unpaired electrons, forming a triple bail. Now all the atoms are happy with their total outer valence shell.
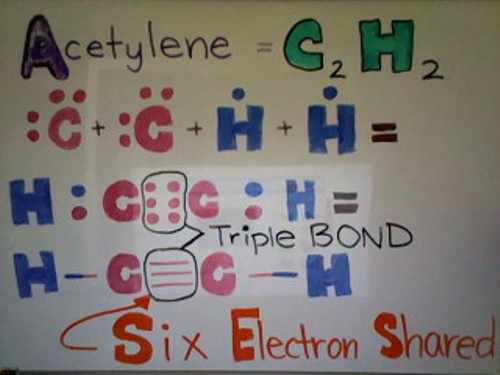
Polar Covalent Bond
A Polar Covalent Bail is created when the shared electrons betwixt atoms are non equally shared. This occurs when one atom has a college electronegativity than the atom it is sharing with. The atom with the college electronegativity will have a stronger pull for electrons (Similiar to a Tug-O-State of war game, whoever is stronger ordinarily wins). Equally a issue, the shared electrons will be closer to the cantlet with the higher electronegativity, making information technology unequally shared. A polar covalent bail will result in the molecule having a slightly positive side (the side containing the atom with a lower electronegativity) and a slightly negative side (containing the cantlet with the higher electronegativity) because the shared electrons will be displaced toward the atom with the college electronegativity. As a effect of polar covalent bonds, the covalent compound that forms volition have an electrostatic potential. This potential will brand the resulting molecule slightly polar, allowing it to form weak bonds with other polar molecules. Ane example of molecules forming weak bonds with each other as a effect of an unbalanced electrostatic potential is hydrogen bonding, where a hydrogen cantlet will interact with an electronegative hydrogen, fluorine, or oxygen atom from another molecule or chemical group.
Example: H2o, Sulfide, Ozone, etc.
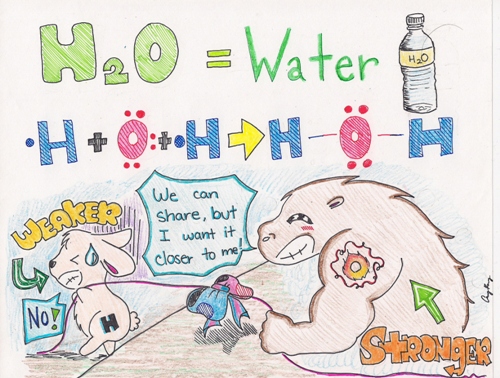
As you tin can run into from the picture in a higher place, Oxygen is the large buff creature with the tattoo of "O" on its arm. The little bunny represents a Hydrogen atom. The bluish and red bow tied in the middle of the rope, pulled past the two creatures represents--the shared pair of electrons--a single bond. Because the Hydrogen atom is weaker, the shared pair of electrons will be pulled closer to the Oxygen atom.
Nonpolar Covalent Bond
A Nonpolar Covalent Bail is created when atoms share their electrons equally. This usually occurs when two atoms have similar or the same electron analogousness. The closer the values of their electron analogousness, the stronger the attraction. This occurs in gas molecules; too known as diatomic elements. Nonpolar covalent bonds have a like concept every bit polar covalent bonds; the atom with the higher electronegativity will describe away the electron from the weaker one. Since this statement is truthful--if we utilize this to our diatomic molecules--all the atoms will have the aforementioned electronegativity since they are the aforementioned kind of element; thus, the electronegativities will abolish each other out and will have a charge of 0 (i.e., a nonpolar covalent bail).
Examples of gas molecules that have a nonpolar covalent bail: Hydrogen gas atom, Nitrogen gas atoms, etc.
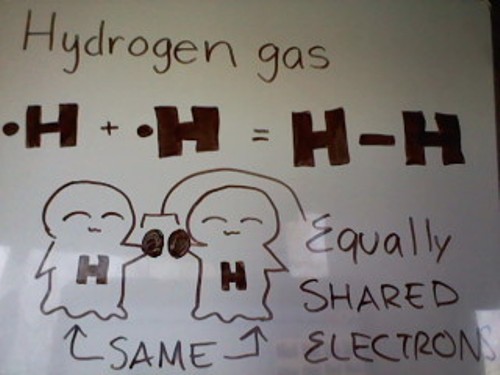
As you lot can come across from the picture show in a higher place, Hydrogen gas has a total of ii Hydrogen atoms. Each Hydrogen atom has 1 valence electron. Since Hydrogen can only fit a max of 2 valence electrons in its orbital, each Hydrogen atom only needs 1 electron. Each atom has i valence electron, so they can simply share, giving each cantlet two electrons each.
References
- Petrucci, Ralph H., Harwood, William S., Herring, F. G., and Madura Jeffrey D. "Full general Chemical science: Principles & Modern Applications." 9th Ed. New Bailiwick of jersey: Pearson Education, Inc., 2007. Print.
- Vaczek, Louis. "The Enjoyment of Chemistry." New York: Viking Press, 1968.
- Pickering, H. S. "The Covalent Bond." London: Wykeham Publications Ltd., 1977.
- Kotz, Treichel, Townsend. "Chemical science and Chemic Reactivity: OWL E-Book Edition." 7th Ed. Ohio: Cengage Learning, 2008.
- Lagowski, J. J. "The Chemic Bail." Boston: Houghton Mifflin Company, 1966.
- Bacskay, George Yard.; Reimers, Jeffrey R.; Nordholm, Sture. "The Mechanism of Covalent Bonding." J. Chem. Educ. 1997 74 1494.
- Reimers, Jeffrey R.; Bacskay, George One thousand.; Nordholm, Sture. "The Basics of Covalent Bonding." J. Chem. Educ. 1997 74 1503.
Bug
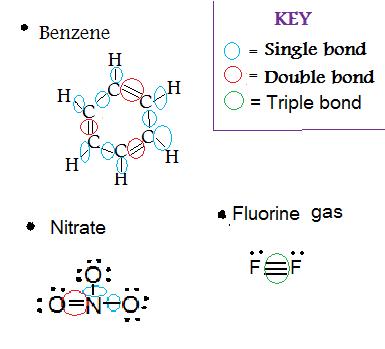
i. Determine the type(s) of bond(southward) in
- Benzene (C6H6)
- NO 3 (Nitrate)
- F2(Fluorine gas)
Solution:
2. Write the electron configuration and determine how many electrons are needed to achieve the nearest noble-gas configuration for the following:
- Arsenic (As)
- Silicon (Si)
- Tellurium (Te)
Solution:

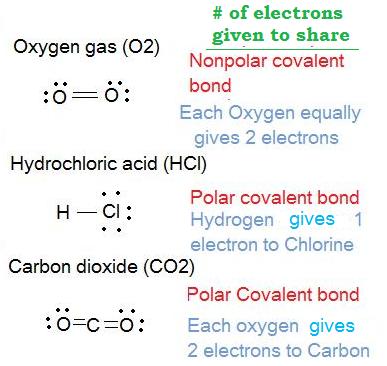
iii. Determine which molecules are polar and which molecules are nonpolar for the following:
- Oxygen gas (O2 )
- Hydrochloric acid (HCl)
- Carbon dioxide (CO2)
Solution:
4. Which of the following statements are true? (There tin be more than one truthful statement.)
- A covalent bond is the same every bit a ionic bond.
- The Octet rule only applys to molecules with covalent bonds.
- A molecule is polar if the shared electrons are equally shared.
- A molecule is nonpolar if the shared electrons are are equally shared.
- Marsh gas gas (CH4 ) has a nonpolar covalent bail because it is a gas.
Solution: But d) is true.
5. Match each atom or molecule with its corresponding letter of the alphabet(s):
- Nitrogen gas
- Argon
- Carbon monoxide
- Hydrogen gas
a) Nonpolar covalent bond
b) Polar covalent bond
c) Follows the Octet Rule
d) Noble gas
due east) Two lone pairs
f) Single bond
Solution:
- Nitrogen gas: a), c), e)
- Argon: c), d)
- Carbon monoxide: b), c), e)
- Hydrogen gas: c), f)
Contributors and Attributions
- Camy Fung (UCD), Nima Mirzaee (UCD)
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Fundamentals_of_Chemical_Bonding/Covalent_Bonds
0 Response to "How Do You Know Where to Put the Covalent Bonds"
Post a Comment